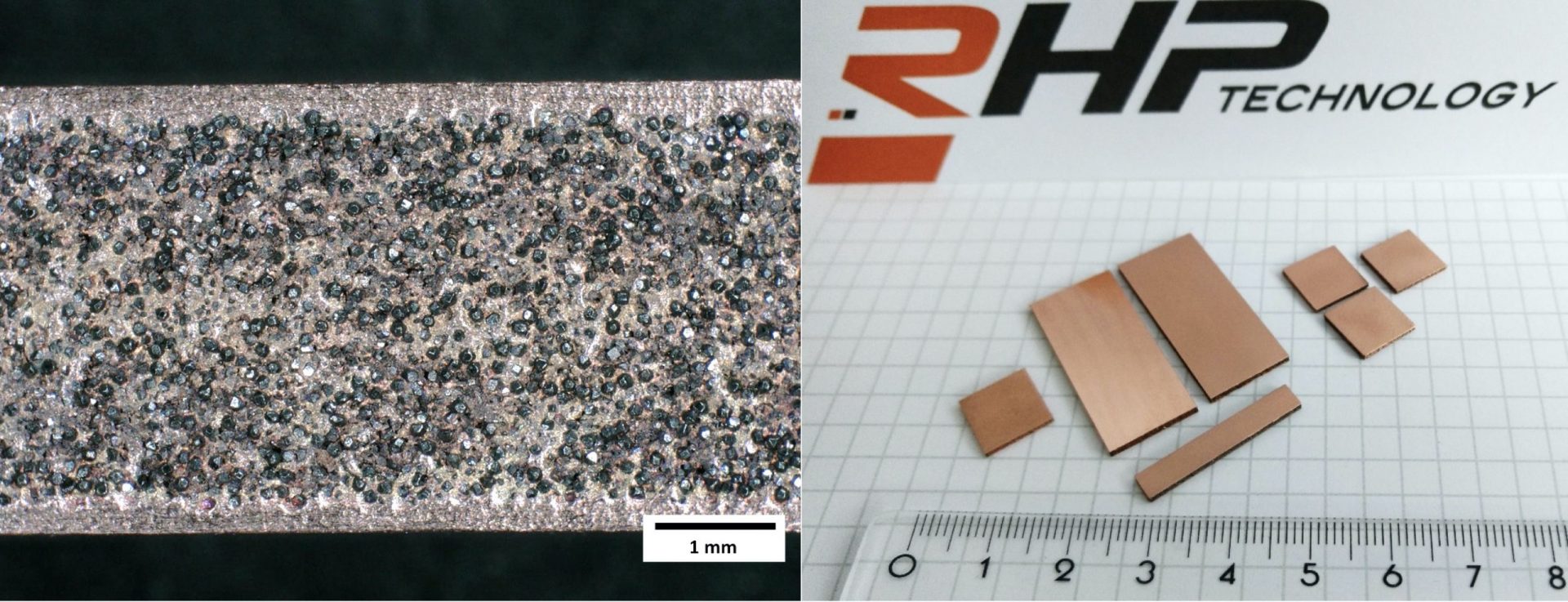
Why diamonds
Diamond is not only a material used in jewelry applications. Due to the very high thermal conductivity (ca. 2000 W/mK) which is 5 times higher than pure copper and its low coefficient of thermal expansion (close to zero) it is also one of the most promising reinforcements for the manufacturing of composite materials with tailored thermophysical properties. Copper or Silver are used as a matrix where diamond particles are embedded. By using powder metallurgical or liquid phase processing it is possible to obtain large composite materials with copper matrix with a thermal conductivity of > 600 W/mK for copper and >800 W/mK for silver based materials.
Typically 50-65 volume percent of synthetic diamonds are used in the composite material in order to take advantage of the excellent thermal properties and to achieve a coefficient of thermal expansion in the range of 6-8 ppm/K which is required to meet the expansion of high performance wide band gap semiconductors such as Gallium Nitride (GaN) in order to avoid a severe mismatch and mechanical stress during thermal cycling.
One of the main issues when working with diamond based materials is the difficulty to shape and machine the material. Appropriate cladding technologies have been developed in order to ensure the manufacturing of high precision parts as well as parts with a good surface finish. Machining technologies have been assessed in order to enable the preparation of high precision parts.